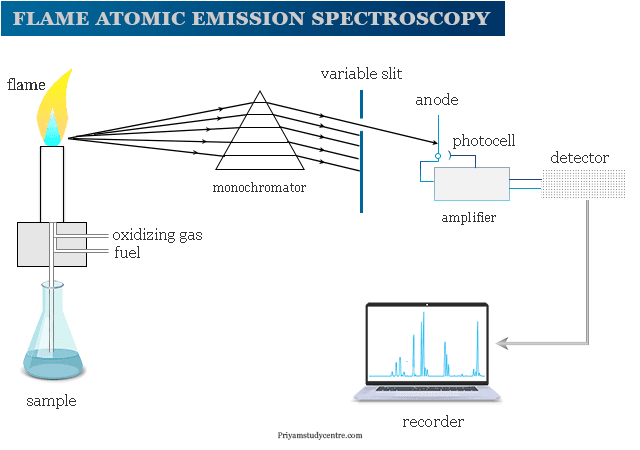
Flame Atomic Emission Spectroscopy
Atomic emission spectroscopy (AES) is a method of chemical analysis of samples by the electronic transition of atoms by using flame and argon plasma sources. The source of excitation influences the intensity of emission in such measurement. The source provides sufficient energy to vaporize the sample. It also causes the electronic excitation of gaseous elementary particles. Therefore, we get band and line spectra. Measurement in atomic emission spectroscopy is feasible because the spectral line has a definite wavelength. Recently, we used inductively coupled plasma atomic emission spectroscopy for the analysis of samples.
Electrons in an atom can stay at a definite energy level. They are called ground state or lowest energy state. When energy is added, one or more electrons are going to a higher energy state by absorption of energy. These excited electrons return to the ground state by radiation of energy.
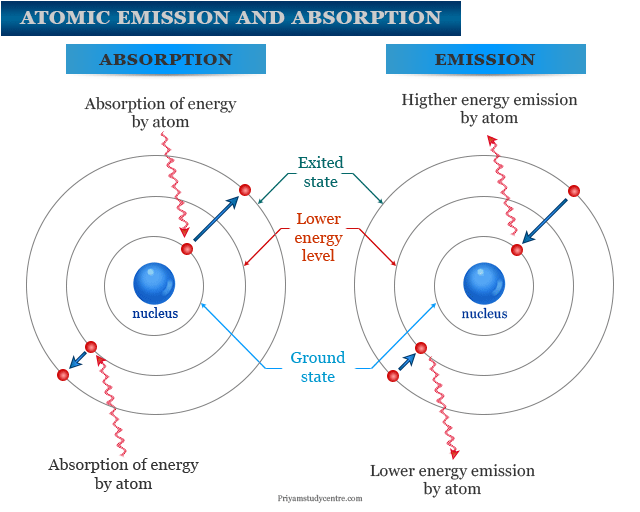
If excitation energy is large then the emitted energy is also large. It forms several lines for atomic emission spectroscopy measurement.
Atomic Emission Spectra
Generally, the flame in the atomic emission spectrometer transforms the solid or liquid samples into a vapour state. It also decomposes the samples into simpler molecules or atoms.
Flame in atomic emission spectroscopy finally excites the electrons to higher energy levels. These electrons return to the ground state by emission of radiation. On dispersing, water or solvent is evaporated and dry salt is left in the flame.
On further heating at a higher temperature, the dry salt is vaporized and molecules are dissociated to neutral atoms which are responsible for emission phenomena.
The vapour of neutral metal atoms is excited by the thermal energy of the flame. A particular element emits characteristic spectra with a definite wavelength. Usually, line spectra are obtained from atoms or ions while molecules give band spectra.
Flame Emission Spectroscopy
Flame emission spectroscopy or flame photometry involves excitation by bringing the gaseous sample into flame or spraying the sample into flame or directly inserting by use of a small loop of platinum wire. Radiation from flame enters a dispersing device to isolate the desired region of the spectrum.
Each element emits light with a characteristic wavelength. It can be dispersed by a grating or prism and detected by a spectrometer.
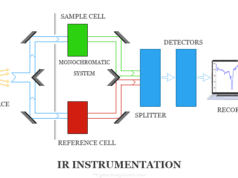
Atomic Emission Spectroscopy Instrumentation
The overall instrumentation of flame atomic spectroscopy is similar to other spectrometric methods like atomic absorption spectroscopy. A flame photometer contains,
- A pressure regulator and flow meter
- An atomizer
- A burner
- An optical system
- A photosensitive detector
- Recording output
- The pressure regulator and flowmeter are used for the proper adjustment of pressure and flow gases.
- The atomizer is used to introduce a liquid sample into the flame at stable and reproducible rates.
- Glycerine can be utilized as a solvent. The burner should produce a steady flame. A Maker burner is good for working at low temperatures. We used a deep metal grid across the mouth of the burner to prevent the flame from striking back.
- The optical system in flame atomic absorption spectroscopy functions as a collector and monochromator of light. It focused on the photosensitive detector. A monochromator can isolate the characteristics of electromagnetic radiation. A good slit with a narrow opening is necessary.
- Photosensitive detectors are not useful because the response is not amplifiable. We used a filter flame photometer in flame atomic emission spectroscopy instrumentation.
- Amplifiers can amplify the signal to boost the output.
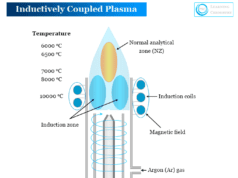
Inductively Coupled Plasma Atomic Emission Spectroscopy
We used inductively coupled plasma in atomic emission spectroscopy for the production of excited atoms or ions. These atoms or ions of a particular element emit radiation with a particular wavelength. Therefore, we used inductively coupled plasma atomic emission spectroscopy (ICP-AES) for better analysis of samples.
Inductively Coupled Plasma (ICP)
Inductively coupled plasma contains a remarkable fraction of electrons and positive ions. It can conduct electricity and is affected by magnetic fields. They are composed of highly energetic and ionized gases produced from noble gases like argon.
They are useful not only for the dissociation of atoms but also for excitation and ionization to give atomic and ionic emission spectra. Argon-supported inductive coupled plasma (ICP) is most common for using atomic emission spectroscopy.
Inductively Coupled Plasma Analysis
Inductively coupled plasma atomic emission spectroscopy can be used in different fields of science and analytical chemistry for the analysis of samples.
- In agriculture, the ICP-AES technique can be used for the analysis of agricultural and food products.
- In earth science, it can be used for the analysis of rare earth elements present in rocks. Trace metals from alloys, steel, lubricating oils, and gasoline can be analyzed by the ICP-AES technique.
- Similarly, in biology, aluminum from blood, copper from the brain tissue, selenium in the liver, and sodium from breast milk can be analyzed by the ICP-AES technique.
- The traces of metals like calcium (Ca), copper (Cu), iron (Fe), manganese (Mn), magnesium (Mg), phosphorus (P), potassium (K), and zinc (Zn) from beer or wine can be analyzed by inductively coupled plasma atomic emission spectroscopy.