Lutetium Element
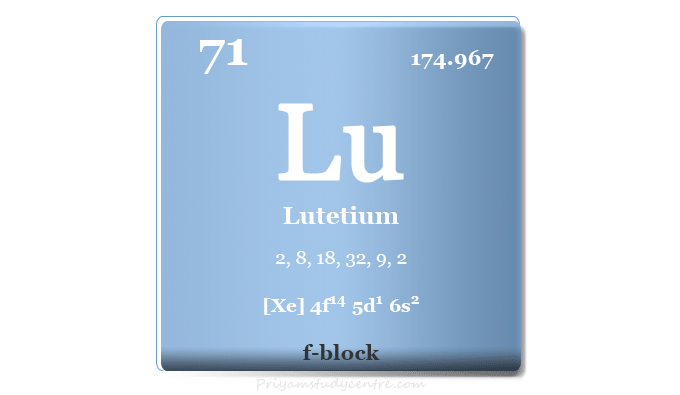
Lutetium is a chemical element or rare earth metal in the periodic table with the symbol Lu and atomic number 71. Due to rare occurrences, production difficulties, and high prices, lutetium is used only for research purposes. Commercially, it is used as a chemical catalyst for creaking hydrocarbons in petroleum refineries. Currently, lutetium-177 is a useful radiometal in radionuclide therapy for the treatment of neuroendocrine tumors and prostate cancer.
Lutetium was discovered independently by French scientist Georges Urbain, Austrian mineralogist Baron Carl Auer von Welsbach, and American chemist Charles James in 1907. The name of the rare earth metal was given from the word Lutetia, the old name of Paris.
Where is Lutetium Found?
The free lutetium metal is never found in the earth’s crust but it is found in almost all other rare-earth minerals. The most common and commercially important mineral of lutetium is monazite. Due to isolation difficulty and very rare abundance, the metal is very expensive to buy.
No lutetium-dominant minerals are found in the earth’s crust. The main mining regions of the metal are China, the United States, Brazil, India, Sri Lanka, and Australia.
Isotopes
Naturally occurring lutetium is a mixture of two stable isotopes with atomic masses 175 to 176. At least 34 radioactive isotopes have been obtained by various artificial nuclear reactions. The element also contains 43 nuclear isomers.
Most of these radioactive isotopes have half-lives of less than half an hour. The primary radioactive decay mode of lutetium isotopes is electron capture or beta decay.
Lutetium-177
Currently, lutetium-177 is the most commonly used radiometal in radionuclide therapy for the treatment of neuroendocrine tumors and prostate cancer. Lu-177 is a medium-energy β-emitter and deposits short-range energy in tissues. It decreases the toxic effects on normal tissues and is suitable for the treatment of disseminated metastatic cancer.
It can be produced in nuclear reactors by direct or indirect nuclear reactions.
- In the direct production process, 176Lu gives 177Lu with some impurity of long-lived 177mLu. It has limited specific activity due to the presence of impurities.
- In the indirect production process, 176Yb irradiates to give 177Lu without impurity of 177mLu. Therefore, it has a highly specific activity.
Properties
Lutetium is a silvery white metal that resists corrosion in dry air but not in humid atmospheres. It is the smallest atom among the lanthanide atoms due to the lanthanide contraction. Therefore, the density, melting point, and hardness of the metal are highest among lanthanides.
| Lutetium | |||
| Symbol | Lu | ||
| Discovery | Georges Urbain and independently by Charles James in 1907 | ||
| Name derived from | The Roman name for Paris means Lutetia | ||
| Common isotope | 71Lu175 | ||
| Oxidation states | +3 | ||
| CAS number | 7439-94-3 | ||
| Periodic properties | |||
| Atomic number | 71 | ||
| Relative atomic mass | 174.967 | ||
| Electron per cell | 2, 8, 18, 32, 9, 2 | ||
| Electronic Configuration | [Xe] 4f14 5d1 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1663° C, 1936 K | ||
| Boiling point | 3402 °C, 3675 K | ||
| Molar heat capacity | 27.20 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 9.84 g/cm3 | ||
| Heat of fusion | 22 kJ mol−1 | ||
| Heat of vaporization | 414 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.24 Å | ||
| Covalent radius | 1.74 Å | ||
| Electronegativity | 1.00 (Pauling scale) | ||
| Electron affinity | 32.81 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 523.52 | 1341.10 | 2022.27 | |
Lutetium in the Periodic Table
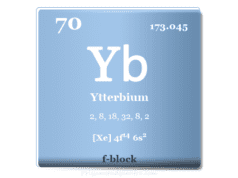
The rare earth metal lutetium is placed in the f-block of the periodic table. It is the last member of the lanthanide series that lies after ytterbium.
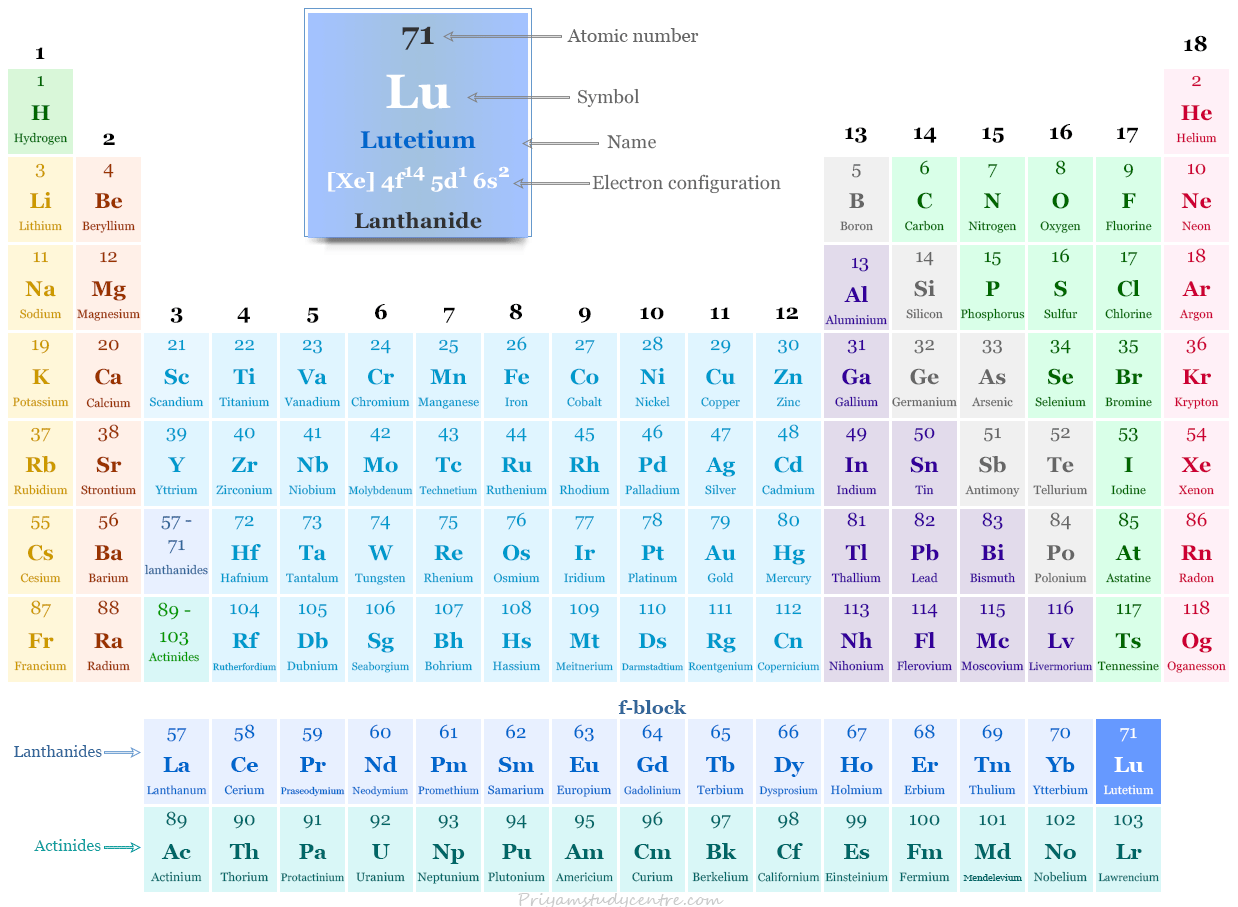
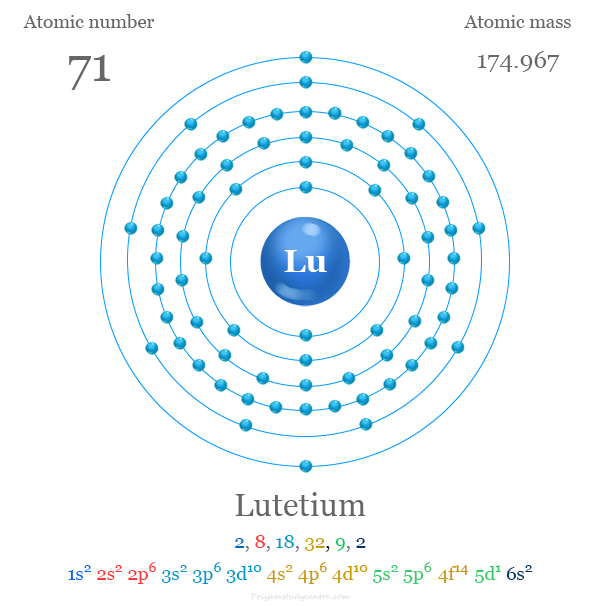
Electron Configuration of Lutetium
The 71 electrons of the lutetium atom are distributed in different energy levels to show the following electronic configuration given below the picture,
Chemical Properties
Like most other lanthanides, it usually shows a +3 oxidation number or state but compounds with 0, +1, and +2 states are also found. Except for iodide, aqueous solutions of most lutetium salts are colorless and crystalize to form white crystalline solids.
It is slightly unstable in dry air under ordinary conditions but rapidly dull in humid atmospheres. It readily reacts with oxygen at 150 °C to form Lu (III) oxide Lu2O3. The resulting oxide is an absorbing agent for water and carbon dioxide.
4 Lu + 3 O2 → 2 Lu2O3
It reacts slowly with cold water and rapidly with hot water to liberate hydrogen and the formation of Lu (III) hydroxide.
2 Lu + 6 H2O → 2 Lu(OH)3 + 3 H2
It forms trihalide salts when reacts with halogens like fluorine, chlorine, bromine, and iodine. Except for the fluoride, all halide salts are soluble in water.
2 Lu + 3 X2 → 2 LuX3 (X = F, Cl, Br, I)
It is readily dissolved by strong mineral acids like sulfuric acid, nitric acid, or hydrochloric acid. In dilute sulfuric acid, it forms a colorless solution that contains the colorless Lu+3 ions.
2 Lu + 3 H2SO4 + 18 H2O → 2 [Lu(H2O)9]3+ + 3 SO4−2 + 3 H2
Facts About Lutetium
- It was the last natural rare earth element that was discovered by three scientists working independently from each other. The credit for discovery was given to Georges Urbain because his results were published first.
- It is the hardest element that contains the smallest atomic size among all lanthanides.
- Lutetium-177 is the most commonly used metal isotope that is used widely for neuroendocrine tumors and prostate cancer.
- The price of lutetium is very high due to its extraction difficulty and rare occurrence.
- It does not play any biological role in human beings with very low toxicity.
Uses of Lutetium
Lutetium is little used outside research due to its rare occurrence, production difficulty, and high price. It has very few commercial uses.
- Lutetium can be used as a chemical catalyst for cracking hydrocarbons in petroleum refineries.
- It may also be used for alkylation, hydrogenation, and polymerization processes.
- It is doped to gadolinium gallium garnet and is used in magnetic bubble memory devices.
- The isotope, Lu-176 has been used as a pure beta emitter due to its half-life and radioactive decay mode.
- Currently, cerium-doped lutetium oxyorthosilicate is used as a detector in positron emission tomography.
- The radioactive lutetium isotopes are used in targeted radionuclide therapy for neuroendocrine tumors.
- Currently, lutetium-177 is the most commonly used radiometal in radionuclide therapy for the treatment of neuroendocrine tumors and prostate cancer.
- Various types of research suggest that Lu-ion atomic clocks provide greater accuracy than any existing atomic clock made by cesium or rubidium.
Price of Lutetium
The pure metal is very difficult to prepare. Therefore, pure lutetium is one of the rarest and most expensive rare earth metals with an average price of US$10,000 per kilogram. The average price of pure lutetium is one-fourth of the price of gold.