Acids
Acids in Chemistry
Acids in chemistry are defined as hydrogen-containing substances that give hydrogen ions (H+) in a water solution. An acid is sour in taste and changes the colour of blue litmus to red. Acids can also has properties to change the colour of the indicators (phenolphthalein, methyl orange, etc) when added to it. They react with metals like iron, zinc, and aluminum to liberate hydrogen.
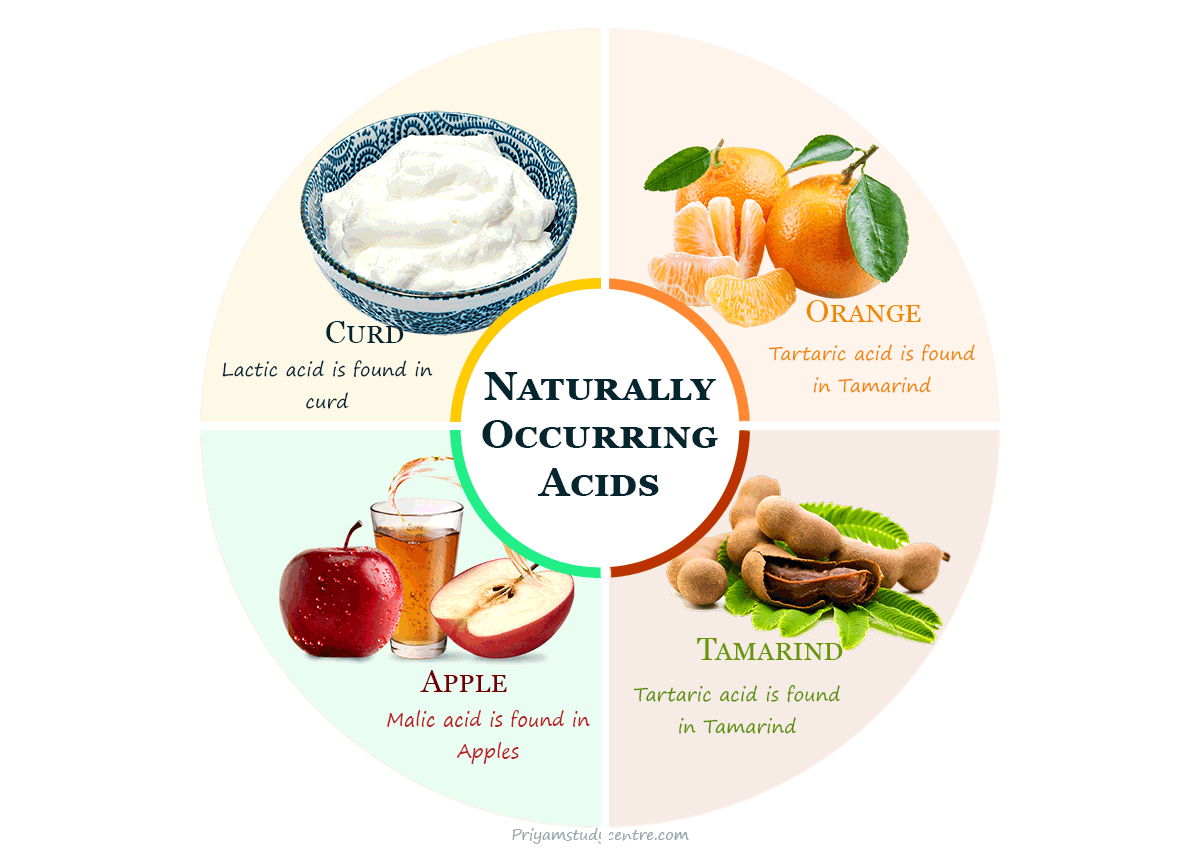
Examples of some naturally occurring acids and their sources are given below in the table:
| Natural Source | Acid | Chemical Formula |
| Vinegar | Acetic acid | CH3COOH |
| Orange and Lemon | Citric acid | C6H6O7 |
| Tamarind | Tartaric acid | C4H6O6 |
| Tomato | Oxalic acid | C2H2O4 |
| Curd | Lactic acid | C3H6O3 |
| Ant sting or bite | Methanoic acid or formic acid | HCOOH |
| Apple | Malic acid | C4H6O5 |
- Most of the chemical compounds have some acidic or basic properties. Strong acids are neutralized by certain types of strong bases to form salts and water. Most of the digestive fluids of humans and animals show some acidic components. For example, HCl present in the stomach helps in the digestion of food.
- Hydrochloric acid (HCl), sulfuric acid (H2SO4), formic acid (HCOOH), acetic acid (CH3COOH), and nitric acid are the most common examples that we use widely in the chemical laboratory and various industries.
They also occur in some naturally occurring fruits such as mango, lemon, orange, tamarind, tomato, etc.
Theories of Acids and Bases
Arrhenius’s definition of acids and bases is limited to aqueous solutions only. However, modern acid-base theories are not only limited to aqueous or water solutions.
Today, these theories can used to define acids and bases in aqueous and non-aqueous solutions or even in a free state. The modern concepts or theories are:
- Solvent System Concept
- Brønsted–Lowry Theory
- Lewis Theory
- Hard Soft Acid-Base (HSAB) Theory
In this part of learning chemistry, we study definitions and acidic and basic properties using different concepts or theories. The various chemistry articles and topics related to acids and bases definitions, properties, indicators, reactions, and practice problems are given below:


