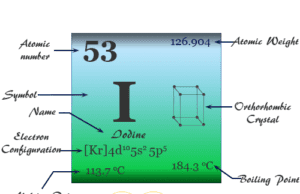
Iodine
Iodine Element
Iodine is a chemical element of Group 17 (VIIA) of the periodic table. It is a member of the halogen family with atomic...
Bromine
What is Bromine?
Bromine is a chemical element of Group 17 (Group VIIA) or the halogen family of the periodic table with atomic number 35 and...
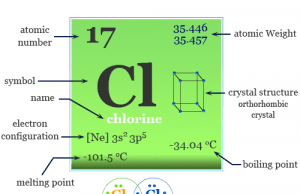
Chlorine
What is Chlorine?
Chlorine is a nonmetal or chemical element of group-17 or the halogen family of the periodic table with the symbol Cl and...
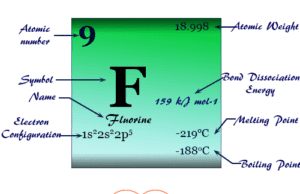
Fluorine
Fluorine element
Fluorine (chemical symbol F), chemical formula F2, atomic number 9 is the most electronegative and most chemically reactive member of the halogen family...